Thyroid Eye Disease
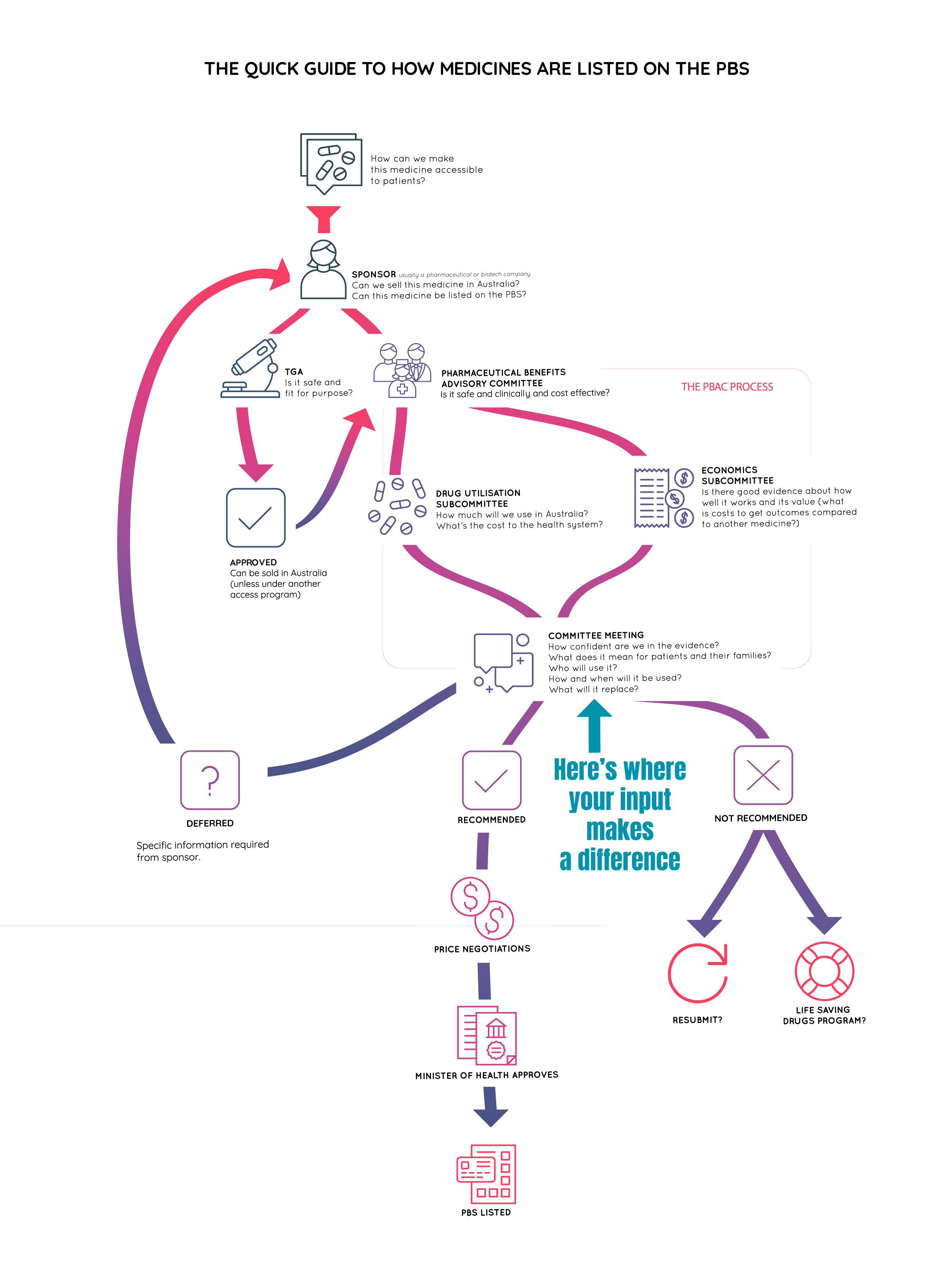
How medicines become available in Australia
The process explained and why your input matters
When you live with a chronic health condition, such as thyroid eye disease (TED), lupus or diabetes, you likely rely on medicines to help you function better. However, some conditions have many medication options available while others don’t have any. That’s mostly because we have been studying common conditions for many decades and they get more research funding. Less common conditions, like TED (also called Graves’ eye disease), haven’t had as much of the spotlight so they have fewer treatment options.
While there are several forms of immunotherapy medicine used in Australia to help people with TED, they were all developed to treat other conditions. However, a new immunotherapy medicine developed specifically to treat TED is currently being considered for approval by the Australian Government. If successful, it will be heavily subsidised through the Pharmaceutical Benefits Scheme (PBS) and made available on prescription.
The following overview shows how the approval process for new medicines works. We’ll also explain how your input can help get new medicines for TED and other conditions available at your local pharmacy.
About the Therapeutic Goods Administration
Before therapeutic goods (including medicines, vaccines, medical devices and products such as vitamins) can be sold in Australia, they must be approved for sale by the Therapeutic Goods Administration (TGA). The TGA is part of the Australian Government Department of Health. The process is referred to as “health technology assessment” (HTA).
Individuals or organisations, such as pharmaceutical companies, can apply for (or “sponsor”) a therapeutic good to be considered for sale in Australia by the TGA. Sponsors must also supply evidence of their product’s quality, safety and efficacy gathered through clinical trials. The submission is then evaluated by the TGA.
If approved, the product is added to the Australian Register of Therapeutic Goods as a “listed” or “registered” product according to its risk profile. No medicine is 100 per cent risk-free, but the risk is greater for some medicines.
Listed products are relatively low risk so they can be sold through supermarkets or pharmacies without a prescription. Not all listed products are evaluated by the TGA for efficacy.
Registered products have a higher risk so they are always evaluated for efficacy and have more rigorous controls placed on them before they go on sale. Some are available in pharmacies over the counter after consultation with a pharmacist. Prescription products are only available with a valid prescription from an approved health professional and can only be sold through pharmacies.
Products that are on the Australian Register of Therapeutic Goods but are not included on the PBS may still be sold in Australia but they are not subsidised by the Government.
About the Pharmaceutical Benefits Scheme
The PBS heavily subsidises the cost of prescription products for all Australians with a Medicare card as well as some Australian visitors. For example, the actual cost of some medications for autoimmune arthritis is well over $1,000 but, through the PBS, consumers pay less than $50 for them at the pharmacy.
Sponsors can apply for registered products to be added to the PBS. These applications are considered by the Pharmaceutical Benefits Advisory Committee (or the PBAC). The PBAC is an independent body of health professionals and consumer representatives appointed by the Australian Government.
The PBAC considers a range of factors when considering new applications, including:
- Submissions from patients and other members of the public about the importance of accessing new treatments
- The number of people likely to use the product.
- The evidence about its safety and efficacy.
- The overall cost to the health system.
- The expected benefits compared to those of comparative existing products.
The PBAC meets at least three times a year to review the applications on their agenda. They assess relevant information from the sponsor, the TGA and public submissions before deciding if they should recommend products to the Minister for Health for inclusion on the PBS.
Public submissions can come from patients, carers, members of the public, health professionals or consumer groups and are a valued part of the HTA process. They help the PBAC understand the importance of new treatments and how the proposed product will benefit patients in their daily lives. These perspectives may be quite different from those presented by the sponsor.
Making your voices heard
At GHLF Australia, part of our mission is to advocate for improved access to health care for people with serious, and often lifelong conditions. One of the ways we do this is by asking members of our community for their insights on how their conditions affect their daily lives and the difference effective treatments can make. For example, patients might describe the physical, mental, social or financial effects of having symptoms that are not well controlled and how a proposed treatment could help them.
We then submit these insights to the relevant government decision-making bodies.
We also teach people how to write their own submissions or share their stories with the government in other ways. Personal testimonies are especially helpful for highlighting the need for, and impact of, innovative new treatments for conditions with little to no dedicated treatments. These can be the difference between treatments being subsidised or not.
(Find out how you can have your say about future treatments for TED and other conditions.)